When you pick up a prescription and see a different name on the bottle - maybe it’s no longer "Lipitor" but "atorvastatin" - you might wonder: is this the same thing? The answer isn’t just yes or no. It’s backed by science, strict testing, and a system designed to keep you safe. That system is called bioequivalence.
What Bioequivalence Really Means
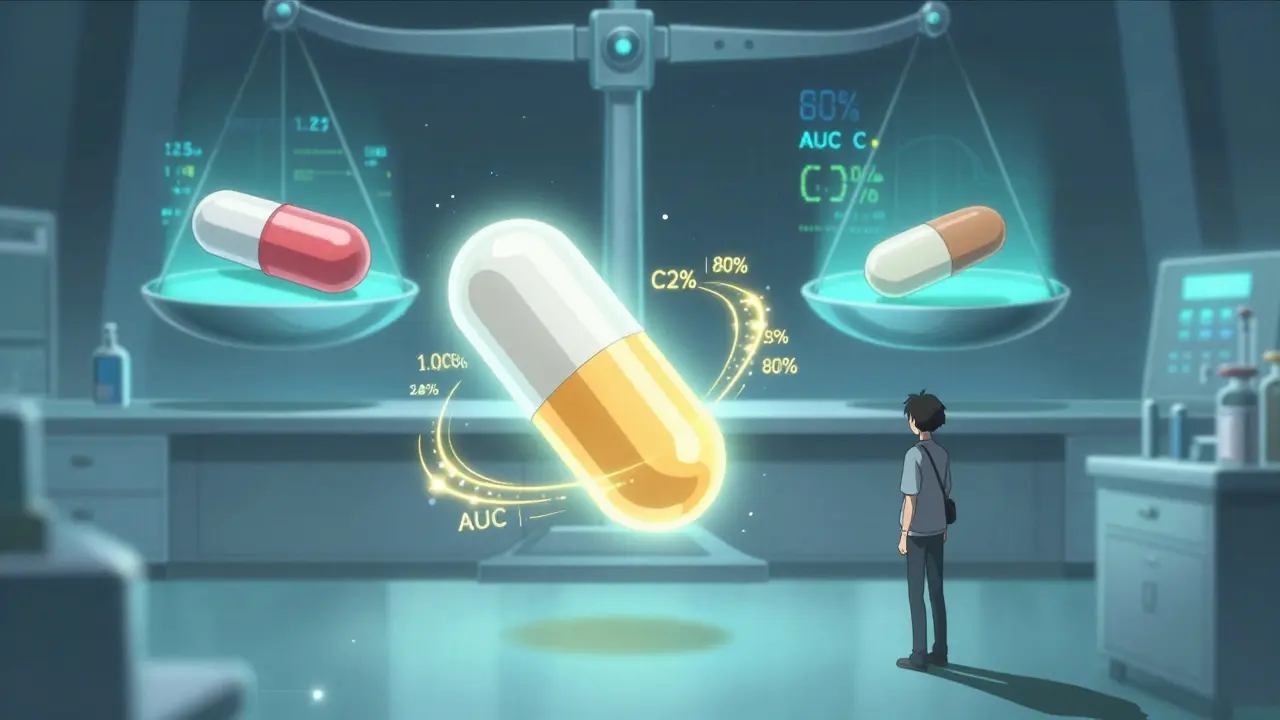
Bioequivalence isn’t about matching pill color or shape. It’s about proving that a generic drug releases the same amount of active ingredient into your bloodstream at the same speed as the brand-name version. If your body doesn’t get the same dose, at the same rate, the drug won’t work the same way. That’s dangerous.The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require that generic drugs show bioequivalence before they can be sold. The standard? The 90% confidence interval for two key measurements - how much of the drug enters your blood (AUC) and how fast it peaks (Cmax) - must fall between 80% and 125% of the brand-name drug’s values. That might sound technical, but here’s what it means in real terms: your body absorbs the generic version almost identically to the original. Not close. Almost identical.
This isn’t a guess. It’s measured in clinical studies, usually with healthy volunteers who take the brand drug and the generic in separate sessions, with blood samples taken over hours to track drug levels. These studies are tightly controlled, with strict rules on diet, timing, and medical screening. For drugs with a narrow therapeutic index - like warfarin, levothyroxine, or lithium - the range is even tighter, sometimes 90% to 111%, because even small differences can cause serious side effects or treatment failure.
Why This Testing Protects Your Health
Imagine switching from your brand-name antidepressant to a generic and suddenly feeling more anxious, dizzy, or tired. That’s what patients fear. And while most people don’t have issues, those rare cases are why bioequivalence testing exists - not to prevent all complaints, but to prevent systemic failures.The FDA tracks adverse events through its FAERS database. From 2020 to 2023, only 0.07% of all reported adverse drug events involved generics that had passed bioequivalence testing. Compare that to 2.3% for brand-name drugs. That doesn’t mean generics are riskier - it means the testing works. It filters out products that could cause harm.
When the FDA tightened bioequivalence standards for levothyroxine in 2012, it was because small changes in thyroid hormone levels can cause heart problems, weight gain, or depression. After the new rules, patient reports of issues dropped significantly. That’s the power of precise testing.
Even when patients report problems after switching - like on Reddit threads or pharmacy forums - pharmacists and regulators look at the data. Are dozens of people having the same issue? Is there a pattern? If not, it’s likely individual sensitivity, not a bioequivalence failure. The system is built to catch real problems, not random anecdotes.
Not All Drugs Are Created Equal
Bioequivalence rules apply to small-molecule generics - pills, capsules, injections. But they don’t apply to biologics. That’s a big difference.Biologics - like insulin, Humira, or Enbrel - are made from living cells. They’re too complex to copy exactly. So instead of bioequivalence, regulators use a different path: biosimilarity. That means comparing structure, function, immune response, and clinical outcomes across multiple studies. It’s more expensive and time-consuming, which is why biosimilars cost less than brand-name biologics, but not as much as traditional generics.
Then there are complex drug products: inhalers, creams, eye drops. For these, measuring blood levels doesn’t tell the whole story. The drug needs to reach the right spot - your lungs, your skin, your eye. That’s why the FDA and EMA are updating guidelines to include new methods like in-vitro testing and advanced imaging. A cream might have the same active ingredient, but if the base formula changes, it won’t absorb the same way. Bioequivalence testing for these products is evolving to match the science.

How Bioequivalence Saves Lives - and Money
Generic drugs make up 90% of prescriptions filled in the U.S., but they cost only 23% of total drug spending. In 2020 alone, they saved the healthcare system $313 billion. That’s not just a number. It means millions of people can afford their meds.Without bioequivalence testing, generics wouldn’t be trusted. Patients, doctors, and insurers wouldn’t accept them. But because the science is solid, people switch without fear. A 2022 survey by the National Community Pharmacists Association found that 87% of patients felt generics worked just as well as brand names. For levothyroxine, 58% of 1,842 reviewers on Drugs.com said it worked the same as the brand.
And it’s not just the U.S. Over 134 countries now require bioequivalence data for generics, up from 89 in 2015. The World Health Organization calls it essential for global access to medicine. In low-income countries, where brand-name drugs are often unaffordable, bioequivalence is the bridge between life and no treatment at all.
The Challenges Behind the Scenes
Getting a generic approved isn’t easy. A single bioequivalence study costs $1-2 million and takes 12 to 18 months. It requires specialized labs, trained staff, and complex statistical analysis.One major hurdle is highly variable drugs - those where the same dose behaves differently in different people. For these, regulators use scaled bioequivalence methods that widen the acceptance range to 75-133%, but only if the drug’s variability is high and the average difference between products is small. This prevents unsafe products from slipping through.
Another challenge? Food. Some drugs are meant to be taken with meals. But testing in fed state is harder - you have to control what people eat, when they eat it, and how long they wait before taking the drug. Japan requires fasting studies even if the brand is taken with food, while the U.S. tests both. That means manufacturers must run multiple studies to sell globally, adding cost and delay.
And then there’s the rise of AI and modeling. The FDA is now accepting physiologically-based pharmacokinetic (PBPK) models to predict how a drug will behave in the body. In 2022, they approved 17 such submissions - up from just 3 in 2018. This could one day reduce the need for human studies, especially for complex products.

What You Should Know as a Patient
You don’t need to understand pharmacokinetics to stay safe. But you should know this:- If your doctor prescribes a generic, it’s because regulators have confirmed it works the same as the brand.
- If you feel different after switching, tell your pharmacist or doctor - but don’t assume it’s the generic’s fault. Most times, it’s not.
- For drugs like warfarin, thyroid meds, or seizure medications, stick with the same generic brand if it works for you. Even with bioequivalence, small formulation differences can affect how your body responds over time.
- Never stop or change your medication without talking to your provider. Bioequivalence ensures safety - but your body still needs monitoring.
Generic drugs aren’t cheaper because they’re lower quality. They’re cheaper because the manufacturer didn’t have to pay for years of research, marketing, and patents. The science behind bioequivalence ensures the quality stays high.
What’s Next for Bioequivalence
The FDA’s 2023 draft guidance now includes specific testing requirements for 1,873 individual drugs - up from 1,422 in 2020. The EMA is updating rules for extended-release products and topical formulations. The goal? Make sure every generic, no matter how complex, meets the same high bar.As more drugs go generic - projected to hit 94% of U.S. prescriptions by 2027 - the system will be tested more than ever. But the foundation remains strong: bioequivalence isn’t just a regulatory checkbox. It’s a promise. A promise that your medication, no matter the price tag, will do what it’s supposed to do - safely and reliably.
Are generic drugs as safe as brand-name drugs?
Yes. Generic drugs must pass strict bioequivalence testing to prove they deliver the same amount of active ingredient at the same rate as the brand-name version. The FDA and EMA require this before approval. Once approved, generics are monitored for safety just like brand drugs. Adverse event reports show that generics approved under bioequivalence standards have far fewer safety issues than brand-name drugs in some cases.
Why do some people say generics don’t work for them?
Some patients report changes in how they feel after switching - like increased anxiety, fatigue, or side effects. But these are usually individual reactions, not signs of bioequivalence failure. The body can respond differently to slight changes in inactive ingredients, like fillers or coatings. For drugs with a narrow therapeutic index (like thyroid or seizure meds), even small differences can matter. That’s why doctors sometimes recommend sticking with the same generic brand. But if you’re having issues, talk to your pharmacist - it’s rarely a problem with the active ingredient.
What’s the difference between a generic and a biosimilar?
Generics are exact chemical copies of small-molecule drugs, like aspirin or metformin. They must prove bioequivalence through blood-level studies. Biosimilars are copies of complex biologic drugs - like insulin or Humira - made from living cells. They can’t be identical, so they must prove similarity through multiple tests: structure, function, immune response, and clinical outcomes. Biosimilars are more expensive to develop and are not interchangeable by default.
Does bioequivalence testing apply to all types of medications?
No. Bioequivalence testing is used for oral pills, injections, and some topical products where blood levels can be measured. But for inhalers, eye drops, or skin creams, the drug doesn’t enter the bloodstream the same way. For these, regulators use different methods - like in-vitro testing, imaging, or clinical endpoint studies - to prove they work the same. The rules are still evolving as science improves.
Can I trust generics if I’m on a narrow therapeutic index drug?
Yes, but with caution. For drugs like warfarin, levothyroxine, or lithium, the acceptable bioequivalence range is tighter - often 90-111% instead of 80-125%. The FDA has updated guidelines specifically for these drugs. If you’re stable on a brand or a specific generic, your doctor may recommend staying on it. But switching between generics that both meet bioequivalence standards is still considered safe. Always monitor your levels and report any changes to your provider.





Amy Ehinger
17 January, 2026 . 06:25 AM
Just switched my levothyroxine generic last month and honestly? Zero difference. I’ve been on it for years and my TSH is still perfect. People freak out over fillers like they’re poison, but my body doesn’t care if it’s coated in cornstarch or cellulose. Just don’t switch brands every month.
Gloria Montero Puertas
17 January, 2026 . 20:23 PM
Let’s be clear: bioequivalence is a statistical illusion. 80%-125%? That’s a 45-point spread! If your drug’s therapeutic window is 10 points wide, you’re gambling with someone’s life. And don’t tell me the FDA ‘monitors’-they’re underfunded, overworked, and let 17 generics through last year with flawed PK models. This isn’t science-it’s corporate convenience.
Tom Doan
19 January, 2026 . 19:31 PM
Interesting. So the FDA’s 80-125% range is statistically valid, yet for narrow-therapeutic-index drugs, they tighten it to 90-111%. That’s not inconsistency-it’s nuance. And yet, we still hear anecdotes about ‘bad generics.’ I wonder if the real issue isn’t the drug, but the expectation that every molecule must behave identically in every human body. We’re biological chaos engines. Maybe we need to stop blaming the pill and start accepting variability.
Sohan Jindal
20 January, 2026 . 14:30 PM
They’re lying. Big Pharma owns the FDA. Why do you think the generics cost so little? Because they’re made in China with cheap fillers and no real testing. My cousin took a generic blood thinner and almost died. They don’t care. They just want you to pay less so they can charge more for the next drug.
Mike Berrange
21 January, 2026 . 07:51 AM
It’s fascinating how people treat bioequivalence like a magic number. The 80-125% range is based on decades of pharmacokinetic modeling, peer-reviewed studies, and real-world outcomes. But when someone says they ‘feel different,’ we jump to ‘the generic is bad.’ Meanwhile, we ignore that their sleep schedule changed, they started drinking grapefruit juice, or their thyroid antibodies are fluctuating. The system works. It’s just that humans hate uncertainty.
Amy Vickberg
21 January, 2026 . 15:04 PM
I’ve been on generic antidepressants for 5 years and honestly? I feel better than I did on the brand. My insurance saved me $400/month. If the science says it’s the same, and my doctor says it’s the same, and I’m not having side effects-why are we even having this conversation? Let people access medicine without financial trauma.
Nishant Garg
22 January, 2026 . 07:11 AM
In India, generics are life. My grandmother takes her warfarin from a local lab-no name brand, no fancy packaging, just a tiny white tablet. But the lab is certified by WHO and their bioequivalence data is published. People here don’t care about the name on the bottle-they care that she’s alive. The West treats medicine like a luxury. Here, it’s a right. And the science? It’s the same. Just less marketing.
Niki Van den Bossche
24 January, 2026 . 05:13 AM
Oh, so now we’re romanticizing bioequivalence like it’s a sacred text? Let’s not forget that the 80-125% range was chosen because it’s statistically convenient, not because it’s biologically perfect. And what about the 12% of generics that fail post-market surveillance? The FDA doesn’t test every batch-just random audits. We’re trusting a system built on trust, not transparency. And don’t get me started on the fact that most bioequivalence studies are funded by the very companies selling the drugs. It’s like letting the fox audit the henhouse.
Crystel Ann
25 January, 2026 . 20:38 PM
My mom’s on lithium. She switched generics three times. Each time, her levels went haywire. Her doctor finally said: stick with the one that works. Doesn’t matter if it’s ‘bioequivalent’-if your body reacts differently, it’s not the same for you. Science doesn’t always account for the messy reality of human biology. Sometimes, consistency beats perfection.
Jan Hess
25 January, 2026 . 23:37 PM
Generics are the unsung heroes of healthcare. No hype. No ads. Just pure science doing its job. And yeah, some people feel weird after switching-but that’s not the drug’s fault. It’s their brain freaking out because the pill looks different. I’ve seen it. Tell someone the pill is blue instead of white and they swear it doesn’t work. The placebo effect is real. So is bioequivalence.
Iona Jane
26 January, 2026 . 13:14 PM
They’re hiding something. Why do the same generics cost 10x more in the U.S. than in Canada? Why do the manufacturers change the filler every 6 months? Why do pharmacies switch them without telling you? This isn’t science-it’s a controlled experiment on the American public. Wake up.
Jaspreet Kaur Chana
27 January, 2026 . 22:03 PM
Back home in Punjab, we call generics ‘jugaad medicine’-clever, cheap, but good enough. My uncle takes his blood pressure meds from a local lab. No name, no logo, just a number. But his BP is stable. The FDA’s standards? They’re great. But they’re not the only way. In places with limited resources, bioequivalence is still the gold standard-just applied with pragmatism. Science doesn’t need a Western label to be valid.
Haley Graves
28 January, 2026 . 13:20 PM
If you’re worried about generics, get your blood levels checked. Simple. No drama. No conspiracy. Just a lab test. If your TSH, INR, or drug concentration is stable, you’re fine. If it’s not, switch back. But don’t let fear of the unknown stop you from saving money and accessing care. You’re not a lab rat-you’re a person with agency. Use it.
Diane Hendriks
28 January, 2026 . 18:26 PM
It’s pathetic. The U.S. allows foreign manufacturers to produce generics under looser standards, then imports them and calls it ‘safe.’ Meanwhile, we’re still paying for the brand-name patents. This isn’t innovation-it’s exploitation. And the FDA? They’re out of touch. They approve drugs based on data from labs that don’t even meet OSHA standards. How is this acceptable?
ellen adamina
29 January, 2026 . 00:15 AM
My pharmacist switched my generic without telling me. I felt weird for two days. Went to the lab. My levels were within 1%. I was fine. Turns out I was just anxious. The system works. Sometimes the problem isn’t the pill-it’s the person holding it.