Pharmacist Substitution Rules: What You Can and Can't Swap Without Knowing
When your pharmacist hands you a different pill than what your doctor wrote on the script, it’s not a mistake—it’s pharmacist substitution rules, the legal framework that lets pharmacists swap brand-name drugs for generic versions under specific conditions. Also known as drug interchangeability, this practice is meant to save money, but it’s not always safe—or even allowed—for every drug. Not all medications can be swapped. Some, like thyroid pills or seizure drugs, need exact dosing. Even tiny differences in how generics are made can throw off your treatment.
These rules are shaped by pharmacy laws, state and federal regulations that control how and when pharmacists can substitute medications. In some states, pharmacists must tell you before switching. In others, they can swap without asking—unless you specifically say no. And if you’re on a psychiatric drug, a biosimilar, or something like levothyroxine, that swap could mean your TSH levels jump, your depression worsens, or your seizures return. Generic drug substitution, the act of replacing a brand-name drug with a chemically similar generic version sounds simple, but the real-world impact isn’t. Studies show patients on substituted psychiatric meds report more side effects and higher dropout rates. That’s not a fluke—it’s a pattern.
It’s not just about the pill. It’s about who controls the switch. Pharmacy benefit managers (PBMs) push for cheaper generics to cut costs, and state laws often side with them. But your doctor’s prescription isn’t just a request—it’s a medical decision. If your script says "do not substitute," that’s legally binding in most places. Yet many patients don’t know that. Others assume all generics are equal, or that their pharmacist would never risk their health. Neither is true. The system is built on trust, but trust isn’t a safeguard. You need to know your rights.
That’s why the posts below cover everything from how to fight a forced switch, to why some drugs like Mestinon or levothyroxine are too sensitive to swap, to how new laws are changing what pharmacists can do. You’ll find real advice on talking to your doctor, checking FDA records, and spotting when a substitution might hurt you. No fluff. No jargon. Just what you need to stay in control of your meds—no matter who’s handing you the bottle.
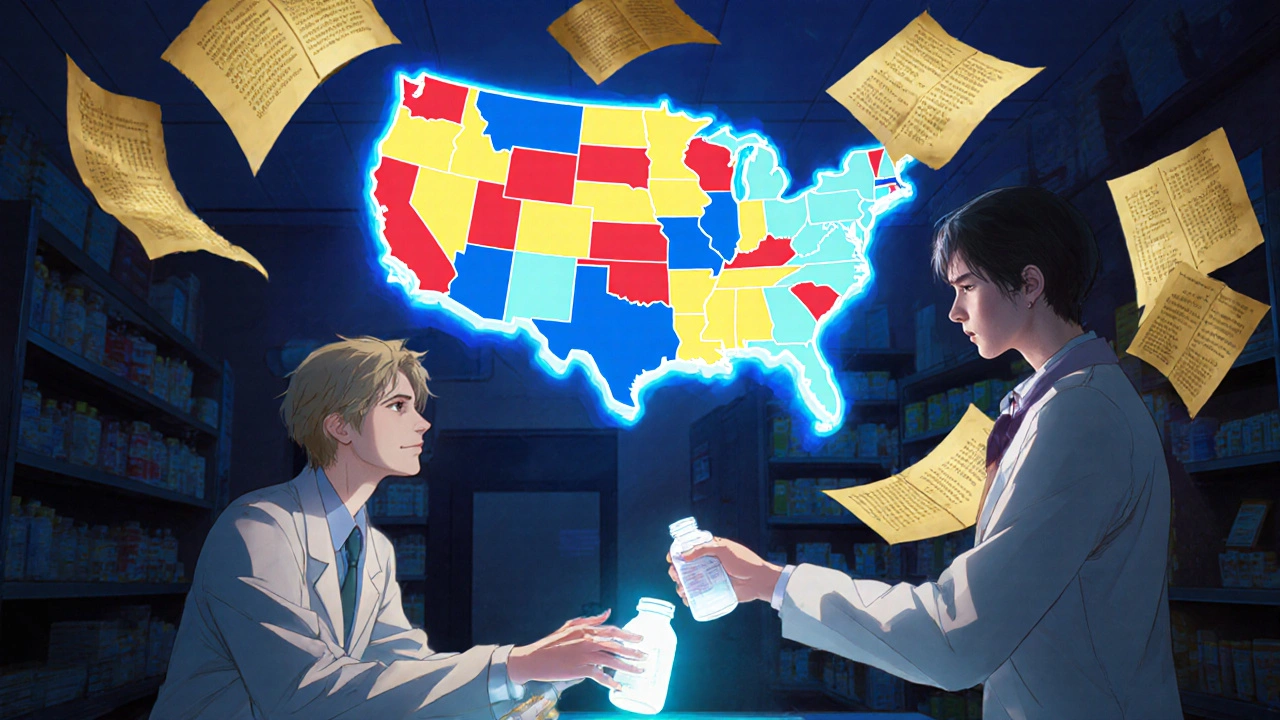
How Generic Substitution Laws Work: State-by-State Breakdown
Generic substitution laws vary widely across U.S. states, affecting how and when pharmacists can swap brand-name drugs for cheaper generics. Learn how each state regulates substitutions, who must give consent, and why some drugs are exempt.
View More