Pharmacovigilance: Understanding Drug Safety Monitoring and Real-World Side Effects
When you take a pill, you trust it will help—not hurt. But drugs don’t always behave the same way in real life as they do in clinical trials. That’s where pharmacovigilance, the science and activities focused on detecting, assessing, understanding, and preventing adverse effects of medicines. Also known as drug safety monitoring, it’s the quiet system working behind the scenes to catch problems doctors and patients might miss. It’s not just about rare side effects—it’s about the muscle pain from statins, the night sweats from antidepressants, the thyroid levels dropping because of acid reflux meds. These aren’t random glitches. They’re signals.
Pharmacovigilance doesn’t wait for a patient to die before acting. It listens to what people report: the woman who can’t climb stairs after starting a cholesterol drug, the man whose hot flashes got worse after switching antidepressants, the person whose blood sugar spiked after adding a new pill. These stories get turned into data. That data feeds into updates for doctors, changes in prescribing guidelines, and sometimes, drug recalls. It’s why you see warnings on labels about interactions with proton pump inhibitors or G6PD deficiency. This isn’t bureaucracy—it’s protection built from real-world experience.
When you look at the posts below, you’re seeing pharmacovigilance in action. Each article ties back to a real safety signal: how generic psychiatric meds can destabilize treatment, why nitrofurantoin can trigger anemia in some, how tenofovir quietly affects teeth, or how steroid doses can trigger psychosis. These aren’t theoretical risks. They’re patterns found because someone spoke up, because a pharmacist noticed a trend, because data was collected and analyzed. Pharmacovigilance turns individual experiences into collective knowledge. You don’t need to be a doctor to understand it—you just need to know what to watch for. Below, you’ll find practical guides on spotting side effects, managing drug interactions, and protecting yourself when medications don’t behave as expected. This isn’t just about knowing the risks. It’s about knowing how to act on them.
International Drug Safety Monitoring Systems Explained
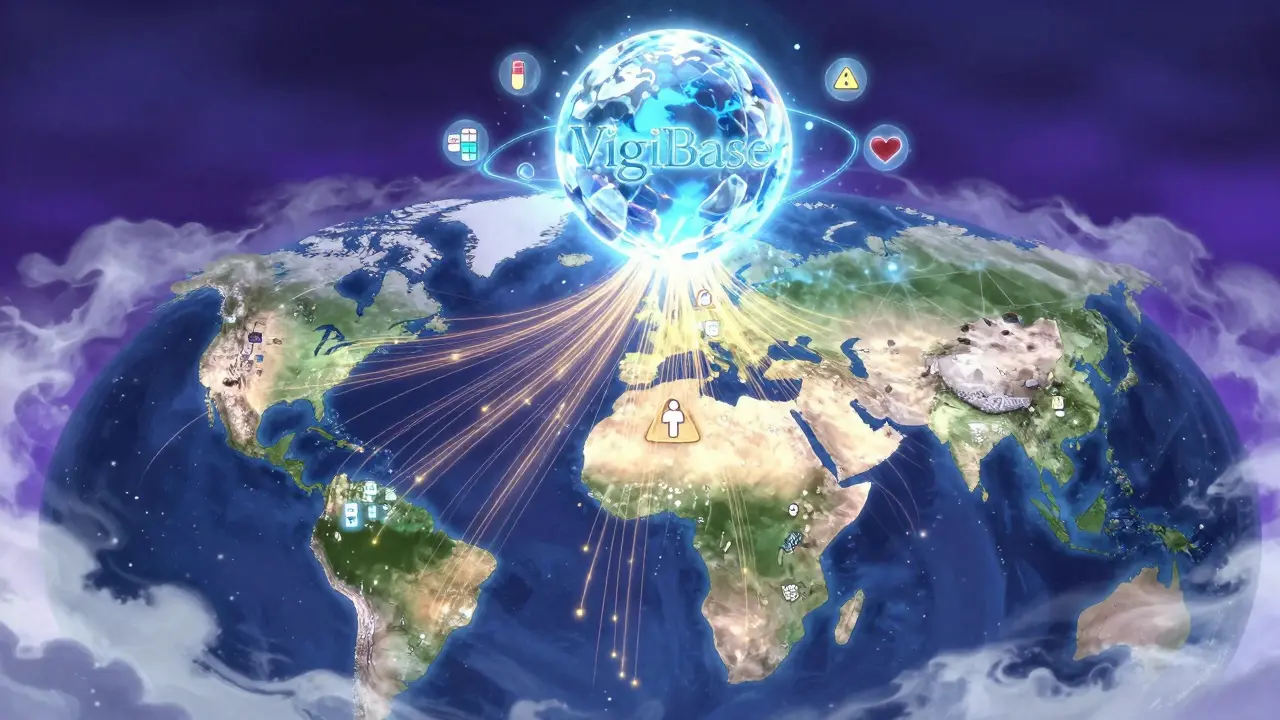
International drug safety monitoring systems track adverse reactions to medicines across 170+ countries using global databases like VigiBase. Learn how pharmacovigilance works, why reporting varies by region, and how technology is improving global drug safety.
View MoreAdverse Event Monitoring for Biosimilars: How Safety Surveillance Works Today
Biosimilars require specialized safety monitoring due to their complex nature. Learn how adverse event tracking works, why product identification matters, and what’s being done to improve biosimilar safety surveillance worldwide.
View More